May 30, 2024
Roslin: Mutation drives bacterial adaptation to immune response
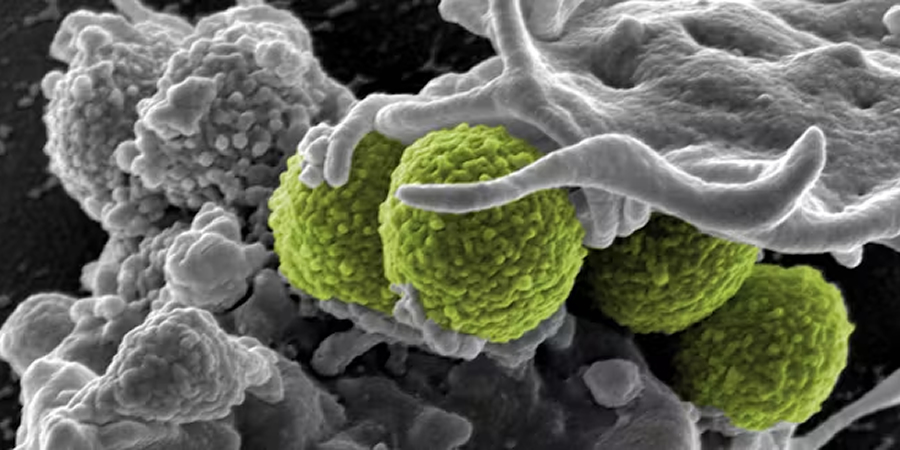
A team of Roslin Institute scientists has developed a model to study how Staphylococcus aureus, a major pathogen associated with human and animal diseases, adapts to antibiotics and immune responses, the institute said.
Researchers designed a framework to repeatedly expose different strains of bacteria to immune cells, known as macrophages, which resulted in changes in bacterial characteristics over time.
The study identified mutations in the bacteria that enhanced several survival traits, such as growth within immune cells and resisting common antibiotics. However, this advantage was conditional and was lost when the bacteria were grown in nutrient-rich conditions outside the macrophage environment.
Using this experimental model in future research could enable scientists to better understand interactions between bacterial pathogens and immune cells, the team said
The physical change, or phenotype, that promoted bacterial survival within immune cells was identified to be a new type of small colony variant (SCV). These variants are associated with a transition to a more persistent and less virulent form of the pathogen, often linked with chronic infections such as osteomyelitis and lung infections in cystic fibrosis patients.
This new SCV adaptation also caused the bacteria to become more resilient against antibiotics such as vancomycin.
The proposed model to repeatedly expose grow bacteria with macrophages can shed light on the conditional nature of bacterial adaptation to specific niches.
Furthermore, understanding bacterial adaptation to evade the immune system could provide insights into potential treatment strategies for infectious diseases in humans and animals, Roslin said.
"Our study uncovers a novel adaptation strategy by S. aureus in response to immune challenges, highlighting the remarkable ingenuity of pathogens in evading host defences," said research fellow Dr. Joana Alves. "Our findings demonstrate the power of experimental models to unravel the complex mechanisms underlying bacterial adaptation during infection."
"We've identified this novel fitness trait that highlights the potential of our model for identifying adaptive traits that emerge during infection," said Prof Ross Fitzgerald, Personal Chair of Molecular Bacteriology. "Understanding the key interactions between bacteria and the immune system can pave the way to new interventions and treatments for infections."
- The Roslin Institute










